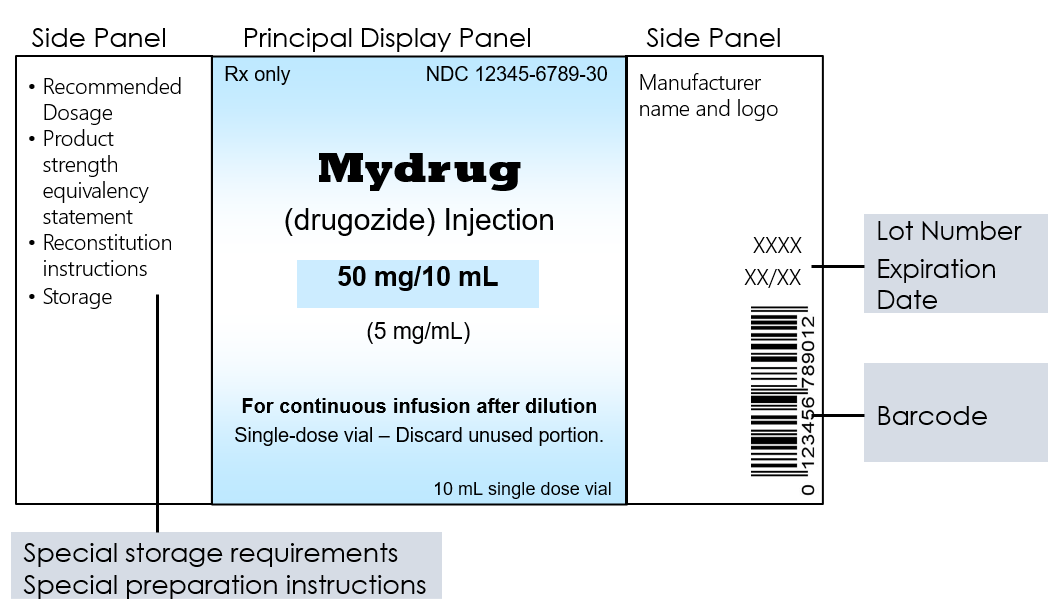
Carton and Container Labeling Resources
FDA’s carton and container labeling specific resources on this webpage are primarily directed to industry staff who develop carton and container labeling for prescription drugs.* For other prescription drug labeling resources for industry such as those for the Prescribing Information, FDA-approved patient labeling, generic drug labeling, biological product labeling, labeling databases, and product databases visit FDA’s Labeling Resources for Human Prescription Drugs. If you are a healthcare professional, patient, or caregiver, visit Frequently Asked Questions about Labeling for Prescription Medicines.
General Carton and Container Labeling Statutory/Regulatory Provisions
- Section 201(k) and (m) of FD&C Act: Statutory definition of “label” and “labeling”, respectively.
- 21 CFR 201.100(b): Prescription label requirements
- 21 CFR 201.10(i): Small label requirements
- 21 CFR 201.6: Misleading statement
- 21 CFR 201.15: Prominence of required label statements
- 21 CFR 610.60: Container label for biological products
- 21 CFR 610.61: Package labeling for biological products
- 21 CFR 610.62: Proper name; package label; legible type
- 21 CFR 610.63: Divided manufacturing responsibility to be shown
- 21 CFR 610.64: Name and address of distributor
Specific Container Label Statement Requirements
- 21 CFR 201.50: Statement of identity (established name)
- 21 CFR 201.51: Declaration of net quantity of contents
- 21 CFR 201.55: Statement of dosage (e.g., Recommended Dosage: see Prescribing Information)
- 21 CFR 201.10: Statement of ingredients (active and inactive)
- 21 CFR 201.17: Location of expiration date
- 21 CFR 201.18: Significance of control numbers (Lot number)
- 21 CFR 201.1: Name and place of business of manufacturer, packer, or distributor
- 21 CFR 201.2: National Drug Code (NDC)
- 21 CFR 201.15: Prominence of required label statements
- 21 CFR 201.20(a): FD&C Yellow No. 5
- 21 CFR 201.25: Bar code label requirements
Carton and Container Labeling Guidances and MAPPs
- Allowable Excess Volume and Labeled Vial Fill Size in Injectable Drug and Biological Products (final guidance)
- Bar Code Label Requirements Questions and Answers (final guidance)
- Best Practices in Development Proprietary Names for Human Prescription Drug Products (final guidance)
- CDER Barcode Inquiries MAPP
- Child-Resistant Packaging Statements in Drug Product Labeling (final guidance)
- Gluten in Drug Products and Associated Labeling Recommendations (draft guidance)
- Harmonizing Compendial Standards With Drug Application Approval Using the USP Pending Monograph Process (draft guidance)
- Incorporation of Physical-Chemical Identifiers into Solid Oral Dosage Form Drug Products for Anticounterfeiting (final guidance)
- Liposome Drug Products: CMC; Human PK and Bioavailability; and Labeling Documentation (final guidance)
- Metered Dose Inhaler and Dry Powder Inhaler Drug Products - Quality Considerations (draft guidance)
- Naming of Drug Products Containing Salt Drug Substances (final guidance and MAPP)
- Product Identifiers Under the Drug Supply Chain Security Act Questions and Answers (final guidance) and Product Identifier Requirements Under the Drug Supply Chain Security Act – Compliance Policy (final guidance)
- Quality Attribute Considerations for Chewable Tablets (final guidance)
- Recommendations for Labeling Medical Products to Inform Users that the Product or Product Container is not Made with Natural Rubber Latex (final guidance)
- Safety Considerations for Container Labels and Carton Labeling Design to Minimize Medication Errors (final guidance)
- Safety Considerations for Product Design to Minimize Medication Errors (final guidance)
- Selection of the Appropriate Package Type Terms and Recommendations for Labeling Injectable Medical Products Packaged in Multiple-Dose, Single-Dose, and Single-Patient-Use Containers for Human Use (final guidance)
- Transdermal and Topical Delivery Systems - Product Development and Quality Considerations (draft guidance)
Other Carton and Container Labeling Resources